Introduction
The term primary
empty sella (PES) is a neuro-radiological finding
defined as an intrasellar herniation of subarachnoid space through a congenital or acquired
defect in the diaphragma sellae, allowing cerebrospinal fluid (CSF) to enter the sella,
which is often associated with stretching of the pituitary
stalk and some degree
of compression and flattening of the pituitary
gland against the sellar floor which can cause various degrees of pituitary
dysfunction in patients without
previous pituitary disorders. The prevalence of PES in the general population varies from 8% to 35% [1,2]. The easier access to the magnetic resonance imaging (MRI), has made PES a frequent incidental finding. Radiologically, the sella is defined as partially
empty when less than 50% of it is filled with CSF
and the pituitary
gland thickness is ≥
3 mm, or totally
empty when
more than 50% of the sella is filled with CSF and the gland thickness is <2 mm in diameter
[3,4]. Even usually
asymptomatic, PES can be associated to serious clinical conditions including different degrees of hypopituitarism and neurological deficits [3]. Several retrospective studies assessed endocrine abnormalities in PES, but few compared
the pituitary functions between total and partial
PES. Moreover, there is no consensual guidelines for the management of PES.
The aim of our study was to analyze
the clinical, hormonal and radiological characteristics of patients
with PES and to demonstrate the correlation between the residual gland volume
and the degree of the hypopituitarism.
Patients and
Methods
The records of thirty-six patients with a diagnosis of PES between
1992 and 2016 seen at the internal
medicine A department of Charles Nicolle’s hospital of Tunis and at the endocrinology department of the military hospital of Tunis were examined retrospectively, 1 year or more after hospital discharge. Anthropometric, historical, endocrine, neurological, ophthalmological and radiological available data of each patient
at diagnosis and during follow-
up were analyzed
and recorded.
Inclusion criteria
All patients having PES diagnosed
by pituitary MRI or computed tomography (CT).
Exclusion criteria
*Subjects with a
previous history of congenital or acquired hypothalamic-pituitary or central nervous system
diseases.
*Subjects with a history of head trauma.
*Subjects with a previous history of medical, neurosurgical, or radiation
treatment for pituitary adenomas or tumors.
*Subjects with ascertained GH and cortisol hypersecretion or patients
with prolactin levels > 100
ng/ml for whom the diagnosis
of an empty sella secondary to the necrosis of a preexisting pituitary adenoma
could not be excluded.
Statistical analysis
All the data were analyzed using
statistic program for Social Sciences (SPSS)
Program Software version 11.5. Data were expressed as mean standard
deviation. The categorical data were assessed
by the Pearson chi-square test. Student’s t and Fisher’s exact tests were used for the comparison of parametric quantitative data. Regression
analysis was used to estimate
the odds ratio (OR) and 95% confidence interval (95% CI) of individual pituitary hormone deficiency between subjects
with total and partial
PES.
Results
Epidemiology and risk factors
A total of 36 patients were included in this study. Population epidemiological and clinical
characteristics are summarized in Table 1. The PES peak incidence
was noted between the 51 and 60 years old. Two male patients
were aged less than 15 years at the inclusion. Among the female patients
with PES,
19 were multiparous. Mean number of pregnancies in the multiparous patients was 5.16 ±1.83. Mean body mass index (BMI) was 30.09 ±7.37 kg/m². Arterial hypertension, type 2 diabetes mellitus
and autoimmune hypothyroidism were found in 38.9%, 25% and 8.3% respectively. Only one patient had idiopathic intracranial hypertension. In our patients, neither primary hypoadrenalism nor primary
hypogonadism were observed. No significant differences
were found among the partial
and total empty sella
subgroups regarding risk factors of PES (table1).
Clinical presentation
Endocrine signs were the most common
presenting symptoms
(52.7%). They were dominated
by hypocortisolism in females and gynecomastia with erectile
dysfunction in males. The two youngest patients presented for delayed growth. PES was found incidentally in 4 cases (11.1%).
More than half of our patients
complained of headache
(52.7%). This headache was variable in localization, severity and duration. It was fronto-orbital, bitemporal or holocranial. The pain was persistent or intermittent and recurrent with a high intensity in most of the cases.
Table 1: Epidemiological
and clinical features of patients with primary empty sella
|
|
n=36
|
Partial PES (n=22)
|
Total PES (n=14)
|
P
|
|
Age
|
47.6 ±15.4
|
49.23±14.9
|
45.14±16.4
|
0.602
|
|
F/M
|
26 /10
|
15 /7
|
11/3
|
-
|
|
BMI
|
30,09±7,37
|
30,25±8,01
|
29,87±6,67
|
0.415
|
|
Multiparity
|
19 (76%)
|
13 (59%)
|
6 (42.85%)
|
0.412
|
|
Obesity
|
15 (41.6%)
|
10 (45.45%)
|
5 (35.7%)
|
0.347
|
|
Overweight
|
7(19.4)
|
2(9.1)
|
5(35.7)
|
0.72
|
|
Hypertension
|
14 (38.9%)
|
11 (50%)
|
3 (21.4%)
|
0.086
|
|
Type 2 DM
|
9 (25%)
|
7 (31.8%)
|
2 (14.3%)
|
0.236
|
|
Headache
|
19 (52.8%)
|
13 (59%)
|
6 (42.85%)
|
0.342
|
Endocrine findings
At least one anterior pituitary hormone deficiency was found in 72% of cases. Ten patients did not have any anterior pituitary deficiency at diagnosis. Secondary adrenal insufficiency was the most common
hormonal abnormality (41.7 %) followed
by hypogonadotropic hypogonadism and central hypothyroidism in 33.3 and
19.4 % of cases respectively. Mild hyperprolactinemia and central diabetes insipidus were also recorded in 19.4% and 5.5% of patients,
respectively. The mean prolactin levels were 47.56
±18.17 ng/ml.
This hyperprolactinemia was isolated in 2 patients. The somatotropic axis was not adequately assessed in adult patients
with PES so we could not rule out this deficiency. An isolated GH deficiency was diagnosed in the two cases of delayed growth with an absence of response
after two GH stimulation tests. In our patients,
the percentage of hypopituitarism in total PES was significantly higher than that in partial
PES (table 2).
The corticotropin deficiency, gonadotropin deficiency and secondary
hypothyroidism were considerably higher in cases with total compared
to the cases with partial PES (OR = 8.0 95% CI 1.7–37, OR = 7.2 95% CI 1.51–34.13,
OR=15.7 95% CI 1.63–152.17,
respectively).
Ophthalmological findings
Visual
disturbances were noted in 12 patients
including decrease
of visual acuity, papilledema
on fundus examination and visual field defects in four, five and three patients,
respectively.
Radiological findings
The diagnosis was confirmed
by CT of the pituitary in 4 patients
and by pituitary MRI in 32 cases. Only one patient underwent both exams. Sixty-one of the patients
had partial empty
sella and the remaining
39% had total empty sella
(Figure 1).
The pituitary stalk was stretched in 3 patients among them only one had central
diabetes insipidus and another had hyperprolactinemia. Other radiological abnormalities on MRI were associated with PES: an absence of the normal posterior
pituitary bright signal in 2 patients consulting for polyuria and an optic chiasma ptosis in a patient with campimetric defect (Figure 2).
Treatment
Medical treatment
The medical treatment
of PES was based on the hormone replacement therapy in case of pituitary
deficiency and pain reliever in case of headache.
Among the patients
with hypopituitarism, early hormone
replacement has been provided
in 79.6% of cases.
The two children with isolated
GH deficiency were submitted to Recombinant human GH treatment was prescribed in two cases. The patients
with symptomatic hyperprolactinemia underwent bromocriptine in four cases
and cabergoline in one case. Objective improvement of symptoms was obtained in
all cases. The two patients with central diabetes insipidus were submitted to
desmopressine intranasal spray treatment at a dose of 25 to 30 µg per
day. That permitted to normalize plasma and urinary osmolarity and serum
electrolytes.
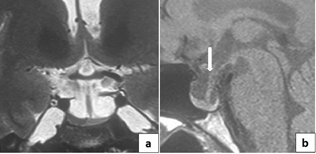
Fgure1: total empty sella with MRI features:
CSF in the sella with pituitary
stalk deviation
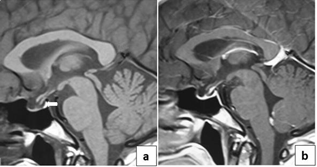
Fgure2: MRI features of total
empty sella with optic chiasma herniation
Table 2: hormonal disorders: partial
vs total PES
|
|
n=36
|
Partial PES (n=22)
|
Total PES (n=14)
|
p
|
|
Corticotropin deficiency
|
15(41.7%)
|
5 (22.7%)
|
10(71.4%)
|
0.005
|
|
hypothyroidism
|
7(19.4%)
|
1 (4.5%)
|
6(42.8%)
|
0.008
|
|
Gonadotropin deficiency
|
12 (33.3%)
|
4 (18.2%)
|
8 (57.1%)
|
0.024
|
|
Hyperprolactinemia
|
7 (19.4%)
|
3 (13.6%)
|
4 (28.6%)
|
0.27
|
Surgical treatment
Only one patient underwent a ventriculo-peritoneal shunt for idiopathic intracranial hypertension (IIH) resistant to medical treatment.
Evolution
Twenty-nine patients
(80.5%) were followed
for an average duration of 4.98 ±4.18 years. Five patients had persistent headache.
Only one patient, initially asymptomatic, complained of headache
appeared after 5 years of follow
up. The anterior
pituitary function was stable in the initially
asymptomatic patients. Only one case of central hypothyroidism was
diagnosed after 6 months
in a woman who had already
a corticotropin insufficiency. Prolactin deficiency was noted after 3 years of follow-up
in a patient with initial
gonadotropic and corticotropin insufficiencies. Imaging regular follow up was performed in 9 patients. It revealed
pituitary thinning with optic chiasm
ptosis in one patient
initially diagnosed with a partial
empty sella. The ophthalmological follow up was performed
in 7 symptomatic patients. It remained unchanged in 3 patients
and revealed decreased visual acuity in 2 patients
and visual field defect in 2 others.
Discussion
PES is a common condition, reported in up to 35% of radiological series,
is typically more prevalent in females
[4]. This entity
pathogenesis is still controversial. Several hypotheses have been proposed,
including a congenital defect of the sellar diaphragm associated with suprasellar increase in the intracranial pressure or volumetric changes in the pituitary gland [5]. Several
studies demonstrated an obvious relationship between obesity and PES. The morbid obesity may induce hypercapnia which causes chronic CSF hypertension. That may lead to the intrasellar herniation of the suprasellar subarachnoid space in patients with hypoplastic diaphragma sellae [6]. Some authors have documented an objective relationship between intra- abdominal, intrathoracic and intracranial pressure in obese patients
[5,6].
Our data are substantially in line with what was reported
in the literature as 41.6% of our patients
were obese. Our results also demonstrated that multiparity may contribute to the development of PES. The enlargement of the pituitary
during pregnancy may lead to weakening of the sellar diaphragm and predispose to intrasellar herniation of cerebrospinal fluid [7]. More than three quarter
of our female cases were multiparous.
Autoimmunity may also have a role in the development of PES that has been suggested
to be a consequence of lymphocytic hypophysitis. Caturegli et al detected
the presence of anti-pituitary hormone antibodies in 22-42% of PES patients
[8].
Other endocrine diseases were described to be associated to PES such as autoimmune primary hypothyroidism, primary adrenal insufficiency and primary hypogonadism. This relationship may be explained by the pituitary hyperplasia seen in case of multiple
hormones deficiencies [9]. In our study, the prevalence of autoimmune hypothyroidism was 8.3%. No cases of primary adrenal insufficiency or primary hypogonadism were found.
PES has been reported
to be associated with several common
endocrine abnormalities. Recent studies have demonstrated that pituitary
hormone deficiency was present in about 8 to 60% of PES cases [10]. Seventy five percent of our patients
had pituitary dysfunction at the PES diagnosis.
The pituitary dysfunction may be due to the chronic compression of the pituitary
gland and the pituitary stalk by CSF. Hyperprolactinemia and GH deficiency have been mentioned as the most prevalent
hormone abnormalities in patients with PES [11]. In our series,
corticotropin deficiency was the most common pituitary
insufficiency. The high incidence
of GH deficiency in patients
with PES might be related to the peripheral disposition of somatotropes within the pituitary gland. That makes these cells
more vulnerable to increased intrasellar pressure. Other authors suggested that obesity might decrease
spontaneous or induced GH secretion [12].
In pediatric series,
GH deficiency represents the most common isolated pituitary deficiency reaching up to 64% of children
with PES [13]. The Hyperprolactinemia is usually
moderate in PES (less than 100
ng/ml). The incidence
is estimated at 10 to
37.5 %. The pituitary stalk compression by CSF may result in a decrease
in the Prolactin inhibiting factor (dopamine). This could explain the hyperprolactinemia in PES patients. However,
the diagnosis of prolactinoma should be kept in mind and evoked for the prolactin levels > 150 ng/ml [14]. In our study, hyperprolactinemia was present in 19.4 %. Among them, only one patient had a stretched pituitary stalk on MRI. Previous reports indicated that hypopituitarism was not related to the residual gland in PES. Our results are concordant with many other studies that demonstrated the correlation between
the residual pituitary
gland volume and hypopituitarism. In all cases of PES, appropriate and exhaustive endocrine assessment should
be done regardless of the type of PES on neuroimaging findings.
Headache is one of the most prevalent symptoms in PES, reported in about 60 to 80 % of cases.
Most of the authors
suggested that pain is due to the traction on vascular-
meningeal structures in the sellar cavity [15]. Visual disturbances have been reported
in only 1.6 to 16 % of cases with PES. The most reported
symptoms are decreased visual acuity and visual field defects (tunnel vision) which usually consists in, bitemporal hemianopsia or quadrantanopia [16]. These troubles could be explained by the intrasellar herniation of the optic nerve or by its decreased blood supply. Severe complications of CSF stasis such as papilledema, optic atrophy or blindness has been reported.
A systematic ophthalmological examination in patients with PES is always recommended.
The treatment of patients with PES includes
appropriate hormonal supplementation in patients
with endocrine dysfunction, dopamine agonists in patients
with symptomatic hyperprolactinemia and pain killers in patients
complaining of headache.
Asymptomatic PES patients are candidate for regular follow up to rule out any regarding hormonal,
ophthalmological disorder
onset [ 17].
Surgical indications for symptomatic PES are controversial and rare. Visual disturbances and cerebrospinal rhinorrhea are the main indications for surgery. CSF shunt placement is effective
to treat PES associated to IIH [18]. In our study, a ventriculo-peritoneal shunt was performed
in an obese patient with IIH resistant to medical treatment. A remission of headache
and papilledema was obtained after surgery. This study highlights PES as progressive disease that induce various hormonal, visual and neurological disorders. The hormonal disorders can appear years after the diagnosis. The gravity of the symptoms
looks related to the residual functional gland volume. A regular
monitoring with clinical, hormonal,
ophthalmological and radiological assessment is mandatory [19,20]. The results of our study should be proved on a larger
comparative prospective trial.
Conclusion
PES should be evoked more often in an obese, multiparous, hypertensive, diabetic woman with symptomatology suggestive of pituitary
dysfunction, chronic headaches or visual disturbances.
The diagnosis is based on hypothalamic-pituitary MRI. The size of the residual
pituitary gland is correlated with the degree of hypopituitarism. Thus, a prompt endocrine, neurologic, and ophthalmologic evaluation at the time of initial
presentation is recommended.
Early hormones replacement therapy relieves
the symptoms and improve the prognosis. Asymptomatic patients should be regularly followed even in the absence
of pituitary dysfunction.
Conflict of
interest
The authors declare that there is no conflict of interest.
References
[1] De Marinis L, Bonadonna S, Bianchi A, Maira G, Giustina
A. Primary Empty Sella. J
Clin Endocrinol Metab. 2005;90(9):5471‑7.
[2] Izizag BB, Ngandu A, Mbiso DL. Empty sella syndrome: a case report. Pan Afr Med J. 2019; 33:317.
[3] Chiloiro S, Giampietro A, Bianchi A, Tartaglione T, Capobianco A, Anile C, et al. Dignosis of endocrine
disease: Primary empty sella: a comprehensive review. Eur J Endocrinol. 2017 ;177 : R275-85.
[4] Lennon MJ, Neuen DR, Suttie
JJ. Partial empty sella in a woman with cerebral
venous sinus thrombosis: A rare presentation of polycythaemia rubra vera. J Clin Neurosci.
2019
; 66 :275-77.
[5] Guitelman M, Garcia Basavilbaso N, Vitale M, Chervin A, Katz D, Miragaya K, et al. Primary empty sella (PES): a review of 175 cases. Pituitary. 2013 ;16 :270-4.
[6] Barzaghi LR, Donofrio CA, Panni P, Losa M, Mortini P. Treatment of empty sella associated with visual impairment: a systematic review of chiasmapexy techniques. Pituitary. 2018; 21:98-106.
[7] Del Monte P, Foppiani
L, Cafferata C, Marugo A, Bernasconi D. Primary "empty sella" in adults: endocrine findings. Endocr J. 2006; 53:803-9.
[8] Caturegli P, Lupi I, Landek-Salgado M,
Kimura H, Rose NR. Pituitary autoimmunity:
30 years later. Autoimmun
Rev. 2008; 7:631‑7.
[9] Arlot S, Lalau JD, Galibert P, Quichaud J. Primary
empty sella turcica.
Analysis of 14 cases and
review of the literature. Ann
Endocrinol (Paris).
1985; 46:99-105.
[10] Auer MK, Stieg MR, Crispin
A, Sievers C, Stalla GK, Kopczak
A. Primary Empty Sella Syndrome
and the Prevalence of Hormonal
Dysregulation. Dtsch Arztebl Int. 2018; 115:99-105.
[11] Zuhur SS, Kuzu I, Uysal E, Altuntas Y. Anterior
pituitary hormone deficiency in subjects with total and partial primary empty sella:
do all cases need endocrinological evaluation? Turk Neurosurg.
2014;24(3):374‑9.
[12] Maira G, Anile C, Mangiola
A. Primary empty sella syndrome
in a series of 142 patients. J Neurosurg. 2005; 103:831-6.
[13] Reetha G, Ravikumar P, Mahesh P. Primary empty sella and associated pituitary
hormonal abnormalities in children: An observational study. Indian J Child Health.2015;2:223‑5.
[14] Ghatnatti V, Sarma D, Saikia U. Empty sella syndrome
- beyond being an incidental finding. Indian J Endocrinol Metab. 2012;16: S321-3.
[15] Agarwal J, Sahay R, Bhadada S, Reddy VS, Agarwal N. Empty sella syndrome. J Indian Acad Clin Med. 2001; 2:198-202.
[16] Dutta D, Maisnam
I, Ghosh S, Mukhopadhyay P, Mukhopadhyay S, Chowdhury
S. Panhypopituitarism with empty sella a sequel of pituitary
hyperplasia due to chronic primary hypothyroidism. Indian J Endocrinol Metab. 2012;16:
S282-4.
[16] Giustina A, Aimaretti
G, Bondanelli M, Buzi F, Cannavò S, Cirillo S, et al. Primary
empty sella: Why and when to
investigate hypothalamic-pituitary function. J Endocrinol Invest .2010;33:343-6.
[17] Aijazi I, Abdullah Al Shama FM, Adam Mukhtar
SH. Primary empty sella syndrome presenting with severe hyponatremia and minimal salt wasting. J Ayub Med Coll Abbottabad. 2016; 28:605-08.
[18] Zetchi A, Labeyrie MA, Nicolini
E, Fantoni M, Eliezer M, Houdart E. Empty Sella Is a Sign of Symptomatic Lateral Sinus Stenosis
and Not Intracranial Hypertension. AJNR Am J Neuroradiol. 2019; 40:1695-1700.
[19] Guinto G, Mercado
M, Abdo M, Nishimura E, Aréchiga N, Nettel B. Primary
empty sella syndrome.
Contemp Neurosurg. 2007; 29:1‑6.
[20] Ortega Lacuesta V, Jara-Albarrán A, Zapata J, Alvarez Hernández J. Empty sella turcica associated with Addison’s disease and early menopause. Med Clin (Barc).
1984; 83:547‑9.
Citation: Belaid R, Khiari K, Ouertani H. Primary empty sella syndrome: Characteristics of the
pituitary deficiency. A bicentric case series.Jr.med.res. 2020; 3(1):3-7.
Belaid et al © All rights are reserved. https://doi.org/10.32512/jmr.3.1.2020/3.7
Submit your manuscript:www.jmedicalresearch.com



