Full Text Article Open
Access 
Original Article
Non tumoral portal vein thrombosis during cirrhosis: Should anticoagulation be proposed?
Bibani Norsaf 1,2, Trad Dorra 1,2*, Bejaoui
Mohamed 1,2, Sabbeh Mariem
1,2, Gargouri Dalila1,2, Elloumi Hela1,2, Ouakaa
Asma1,2, Kharrat Jamel1,2.
|
1: Department of gastroenterology Habib Thameur
Hospital Tunis Tunisia
2: College
of medicine Tunis
Tunisia
*Corresponding author Correspondence to: dorratrad@yahoo.com Publication data: Submitted: January 17,2018
Accepted: March 4,2018 Available Online: June 22,2018
This article was subject to full peer- review.
|
Abstract
|
|
Background:
Portal vein thrombosis (PVT) is considered as infrequent and pejorative event in cirrhosis. Up to date,
many questions remain
about therapeutic management.
Aim:
The objectives of this study were
to assess the impact of the PVT
on the progression of liver
disease, to review the indications for anticoagulation
and its repercussions.
Materials and methods:
A case-control study was conducted over a period
of 12 years (2002-2013). It included 484 cases of cirrhosis. Among these patients, 41 had non tumoral portal vein thrombosis (case group). The
control group
included the remaining 443 patients.
Results:
In our study, there was no impact of
PVT on the natural history
of cirrhosis both
in terms
of complications or survival. Only
the early introduction of anticoagulant therapy was associated with a re-permeabilization of portal
vein at one
year (OR1.6; 95% CI [1.10-2.01]). Prolonged anticoagulation was inversely correlated with recurrent PVT after treatment. However, obtaining a portal
vein re-permeabilization was not correlated to a significant gain in terms
of prevention of complication related to cirrhosis and survival.
Conclusions:
results suggest that portal vein
thrombosis in patients with cirrhosis is not a formal indication for anticoagulant therapy. It should
be reserved for candidates of liver
transplantation, those with an extension of the PVT to mesenteric vessels or with severe
prothrombotic status.
Key words:
portal vein thrombosis, cirrhosis, anticoagulation.
|
Introduction:
Non tumoral
Portal vein thrombosis (PVT) during cirrhosis is considered as an uncommon and pejorative event
[1]. The causes of PVT belong usually to local and / or general factors, including cirrhosis [2]. However,
the impact of PVT on the cirrhosis mortality
and liver disease
progression remains questionable. Therapeutic management of PVT remains
difficult due to the lack of national
and international guidelines
and the absence of objective tools
for benefit -risk balance assessment.
Patients and
methods:
In our work, we first investigated the indications of anticoagulants in a group of cirrhotic patients
with non-tumoral PVT. We studied
efficiency as well as complications occurring during anticoagulation. In a second step, we studied the effect
of PVT on the progression of liver disease and the impact of the re-permeabilization on survival.
A case-control study including all adults with cirrhosis hospitalized in the Gastroenterology department of the Habib Thameur Hospital
during 12 years (January 2002 May 2013) was performed.
The case group consisted in patients
with:
-Cirrhosis diagnosed
most often on the association of clinical,
biological, morphological and endoscopic arguments;
-Acute or chronic PVT diagnosed
by Doppler ultrasound or by tomodensitometry with intravenous contrast;
-A minimum
follow-up of 3 months;
The control group was composed of patients with the same inclusion criteria but without
PVT.
Patients
with a history of neoplastic pathology in remission, or hepatocellular carcinoma (HCC) were not included
in our study.
Exclusion
criteria were:
·
Patients who received anticoagulation for another indication than the PVT before their inclusion;
·
Patients with a follow-up of less than 3 months;
·
Patients who developed a HCC within a period of 6 months next to PVT diagnosis.
The diagnosis of PVT was made by ultrasound coupled with the Doppler or by a tomodensitometry with contrast
injection. The main objective of imaging
was to establish the diagnosis of PVT, to determine its partial or total
character, to specify its extension
in particular to splanchnic vessels and to eliminate mesenteric venous ischemia
Imaging aimed also to eliminate neoplastic causes for PVT as well as septic pylephlebitis.
Endoscopic monitoring was performed
for all patients according to the last Baveno
VI guidelines. Primary
or secondary prophylaxis of gastrointestinal bleeding was established according to endoscopic data. Each time a treatment for PVT has been established, the following
data have been specified: the therapeutic indication,
the modalities of the treatment, the delay in initiating the treatment
with respect to the diagnosis
of PVT and its duration. Clinical
and radiological follow-up of the patients were recorded.
We studied the spontaneous radiological evolution or under anticoagulant treatment, as well as the evolution of the hepatic function according to the re- permeabilization or not of the portal
vein. When a radiological follow-up
was carried out during the year following the diagnosis
of PVT, the reversal of the PVT was qualified as total, partial
or absent.
The success
of the treatment instituted was confirmed by a total re-permeabilization of the portal vein.
Hemorrhagic complications (digestive or extra- digestive) under anti-coagulation were recorded, as well as their time of appearance and their evolution. At the end of the study survival was compared
in both
groups.
The statistical analysis was carried out by SPSS.21.
The averages
were compared using
the Student T test and the Mann and Whitney
nonparametric test. The comparison of percentages on independent series was carried out by the Pearson
Chi-square test and the Fisher test. The survival
analysis was performed according to the Kaplan-Meier method. The analysis
of the prognostic factors was based on the Log-Rank
test for the univariate analysis. A logistic regression according to the Cox model was used for the multivariate analysis.
A p value was considered statistically significant if <0.05.
Results:
A total of 548 cirrhotic patients
hospitalized in the department were recorded.
In total, 484 cirrhotic patients have no HCC, in which 41 cases with PVT and
443 controls were included.
The prevalence of non tumoral PVT in cirrhosis was thus of 8.5% in our study. Twenty-three patients (56.1%)
received anticoagulant therapy.
The indications of anticoagulation were:
· Extension
of the PVT to the mesenteric vessels with or without
signs of intestinal ischemia: 12 patients
(one died before the beginning
of anticoagulation).
· Severe
prothrombotic status (protein C deficiency, anti-thrombin III deficiency): 3 patients (1 case of one extension of the PVT to the mesenteric veins).
· When the benefit-risk balance was in favor of anticoagulant treatment: 10 patients
with mild cirrhosis (CHILD A and B7 score).
All
patients treated (n=23) received Antivitamin K (AVK)-based anticoagulation. The 11
patients with extension of the PVT to the mesenteric vessels with or without
signs of intestinal ischemia as well as the 2 patients with a severe prothrombotic status initially received an anticoagulant treatment based
on low molecular weight
heparin (LMWH) then relayed
by the AVK.
The average
time to introduce AVK was 2.6 days. LMWH was introduced immediately in case of mesenteric ischemia.
The mean duration
of anticoagulation was 8.65 months (1-24).
The average
duration of follow-up was 26.4 months (1-120). Seven patients had no radiological control of their PVT. For the others, Doppler
monitoring was performed
every 3 to 6 months.
Among the 34 patients followed
over 3 months, re- permeabilization
was obtained in 19 cases (55.8%). It was total in 29% of cases. In patients
with anticoagulant therapy (n=23), portal re- permeabilization was obtained in 69.5% (n=16) and was total in 10 (43.5%).
In the 11 untreated patients, re-permeabilization was obtained
in only 27.2% (n=3) and no case of total
re- permeabilization of the portal vein was noted. The difference was statistically significant (p=0.025) (Figure 1). In
the
treated group re-permeabilization was obtained within
a year in 79% of cases.
The average duration
of re-permeabilization was 7.9 months. All patients treated for 12 months (n=10) had complete re-permeabilization of their portal vein. On the other hand, in 9 patients
treated for less than 6 months, a re-permeabilization was obtained in 44.4% of the cases (n=4), and a PVT reappeared in one case. (Figure
2)
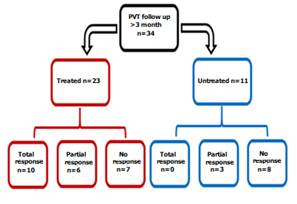
Figure 1: Evaluation of the re-permeabilization of the
portal vein with vs without anticoagulation

Figure 2: time to PVT re-permeabilization
(months)
During the follow-up, 2 patients presented extra-digestive hemorrhage (epistaxis) and one case of gastrointestinal hemorrhage due to varicose
rupture. Two cases of gastrointestinal hemorrhage were recorded in untreated patients (6.5%).
Eleven (32.3%) patients
developed a non-hemorrhagic complication following the diagnosis of PVT including 2 cases of refractory ascites, 5 cases of hepatic encephalopathy and 4 cases of spontaneous bacterial
peritonitis. Overall survival at 1 year and 2 years were respectively 68.3% and 34.1%.
The median survival
was 24 months. At two years, 4 of the
27 patients who died were in the successful group. The remaining 23 were among patients with failure
or absence of the treatment. Liver disease progression was the cause for all the patients
of the treated
group and for 20 patients
from the other group.
Thus, at two years, the overall mortality rates in the two groups were 40% and 74.2% respectively. If only specific mortality is considered, the respective rates
increase to 40% and 64.5%.
The introduction of an anticoagulant treatment but especially its early character (within 30 days after the diagnosis of the PVT) represented decisive factors in the obtaining of a portal re-permeabilization in our study. Thus, 3 factors appeared
to be correlate with portal re-permeabilization in univariate analysis:
initiation of anticoagulant therapy (p = 0.025),
initiation of treatment within one month after diagnosis
of PVT (p = 0.005), and a partial PVT (p = 0.027). However, in multivariate analysis, only the rapid onset of treatment within
7 days was significantly correlated with re-permeabilization of the portal vein with an OR of 1.6; 95% CI [1.10-2.01] (Table 1)
The introduction of effective anticoagulant therapy (with complete portal
re- permeabilization) does not seem to have any effect on the evolution of cirrhosis. Thus, there was
no significant difference between the two groups of patients in case of regression or persistence of PVT
concerning complications such as spontaneous bacterial peritonitis (p = 0.912), refractory ascites
(p
= 0.263), hepatic encephalopathy (p = 0.748), gastrointestinal hemorrhage (p = 0.421). Moreover,
the overall rate of complications was comparable between the two groups (p = 0.452).
Twenty-four liver-related deaths occurred
in the first two years, of which 4 were successfully treated for the PVT. There was no significant difference between
the patients in whom total permeability was obtained
comparing specific
mortality rate at
1 year (p = 0.282)
and at 2 years (p = 0.171) (Figure 3).
|
Variable
|
p (univariate analysis)
|
p (multivariate analysis)
|
|
Treatment
|
0.025
|
NS
|
|
Partial PVT
|
0.027
|
NS
|
|
Initiation of the treatment within 30 days
|
0.005
|
0.038; OR=1.6 [1.1 – 2.01]
|

Figure 3: Survival after
total re-permeabilization obtained (blue
curve) vs not obtained
(red curve)
Three hemorrhagic events
(2 cases of epistaxis
and 1 case of gastrointestinal hemorrhage due to varicose rupture) occurred under AVK. The mean time to bleeding complication was 1.7 months
and the 3 hemorrhagic events occurred during the first quarter of treatment. Two cases
of gastrointestinal hemorrhage occurred
in untreated patients (6.5%). The patients
presenting hemorrhagic events were minor and the AVK treatment
was not discontinued.
Discussion:
The lack of consensual guidelines for the management of PVT in cirrhotic
patients is may be due to the difficult
assessment its impact on the cirrhosis natural history.
However, the basic concept of any proposed
treatment is the safety and the positive impact on the evolution
of the liver disease [3].
Regarding
primary prevention, Villa et al demonstrated that the use of Enoxaparin 4000 IU once daily for 48 weeks in CHILD B7- C10 cirrhotic
patients prevented both the onset of PVT and the decompensation of the cirrhosis (p <0,0001 compared
to controls)
and improved mortality (p=0.02)
with a benefit maintained between
2 and 4 years [4]. This suggests the action
of anticoagulation both on PVT and progression of hepatopathy. Thus, alteration of hepatic and / or intestinal microcirculation seems to be under the direct influence of coagulation
abnormalities.
Previously in the literature; 6 studies
including a total of 199 patients treated this topic (Table
2).
Our results
about the effectiveness of anticoagulation for PVT in cirrhotic patients are limited.
Heterogeneous data; the lack of precision in the assessment of the PVT extension; and unavoidable selection bias in some situations decreased the specificity of the analysis.
However, the tolerance
and the absence
of interference with the mortality due to digestive bleeding
are well demonstrated now. This was also remarkable in many other studies
[4,6,9,10]. For spontaneous or induced (secondary
to paracentesis for example) extra-digestive hemorrhage, the only established risk factor
is severe thrombocytopenia <50,000 / ml [5-10].
|
|
Type of study
|
n (controls)
|
Severity of the cirrhosis
|
Type and duration of anticoagulation
|
Type of PVT (patial/total)
|
Re-permeabilization (total/partial)
|
Stabilization/ progression
|
Bleeding complications
|
|
Werner and al (3)
|
Retrospective
|
28
|
MELD 7-29
|
Warfarin 10 months
|
-
|
11 (39.3%)
/ 17
(60.7%)
|
10(53%)/1(5%)
|
1 vaginal
|
|
Villa and al (4)
|
Controlled randomized essay
|
34 (36)
|
Child Pugh 7-10
|
Enoxaparin 12 to 24 months
|
Primary prevention
|
-
|
7/0
|
4 (2 digestive)
|
|
Delgado and al (6)
|
Retrospective
|
55
|
MELD 12.8
|
Warfarin Enoxaparin
6.8 months
|
41 (75%)
/ 14 (25%)
|
25 (45%) / 30 (55%)
|
0/0
|
10 (8 digestive)
|
|
Amiltrano and al (7)
|
Prospective
|
28
|
-
|
Enoxaparin 6months
|
23 (82%)
/ 5 (18%)
|
21 (75%) / 5 (18%)
|
0
(treated)/10(28%) untreated
|
0
|
|
Senzolo and al (12)
|
Cases, controlled, Prospective
|
33 (21)
|
MELD 12.6
|
Nadraparin 6 months
|
24 (69%)
/ 11 (31%)
|
12 (34%)/
9 (26%)
|
0/2(7%)
|
4 (1 digestive)
|
|
Francoz and al (16)
|
Cases, controlled, Prospective
|
19 (10)
|
MELD 12.8
|
Warfarin 8 months
|
18 (95%)
/ 1 (5%)
|
8 (42%) / 0
|
7(20%)/5(15%)
|
1 (after band ligation)
|
|
Our study
|
Cases, controlled, retrospective
|
41 (434)
|
MELD 15.9
|
Antivitamin
K 8.7months
|
30 (73%)
/ 11 (27%)
|
Treated (n=23) 10/6 Untreated (n=11) 0/3
|
3/5
|
3 (1 digestive)
|
|
|
Table 2: review of previous reports regarding anticoagulation for PVT in cirrhotic
patients
About the curative
treatment, data from the literature agreed with our results for total or partial re-permeabilization (rates are 40% and 15% respectively) [11]. Complete re-permeabilization of the portal vein is obtained
for almost all patients with treatment duration >1 year [7,12] early discontinued treatment is associated with recurrence in 25% of cases [6]. Some other authors
support the fact that 40% of the PVT decreases in size spontaneously. The only predictive factor of re- permeabilization under anticoagulation is, such as found in our study,
the early introduction of anticoagulant. The relationship between permeability and complications, described in some series, was not confirmed by comparative studies [13]. Many new oral anticoagulants have been commercialized, Rivaroxaban® proved its efficacy and safety [14,15]. These molecules have many advantages such as easy route of administration and the absence
of interaction with the INR and MELD score.
Therefore, no continuous monitoring is required.
Their disadvantage includes the absence of antidote and frequent drug interactions.
Finally,
in light of the above publications and our results,
anticoagulant therapy is recommended in the following situations:
1.
In patients
with advanced cirrhosis who are considered for short-term
or
medium-term therapy, an anticoagulant treatment, preceded
by a preventive treatment of gastrointestinal bleeding should be proposed. An easier
access for hepatic transplantation and the improvement of post- operative survival are rational behind this recommendation.
The aim is to solve the portal obstruction or at least to limit its extension.
2.
In the presence of a PVT extended
to the mesenteric vessels with or without signs of intestinal ischemia. The aim of the treatment
is to prevent mesenteric infarction.
3.
A strong
prothrombotic status associated with PVT in a cirrhotic patient is also an indication for anticoagulant therapy alone or in combination with TIPS.
Conclusions:
Our study showed that untreated
PVT has no impact on the progression of cirrhosis neither on the overall survival. the only complication correlated with the portal obstruction was the gastrointestinal hemorrhage with a higher incidence and a more complicated management. From a therapeutic point of view, only the early introduction of anticoagulant therapy was associated with portal re-permeabilization at one year and prolonged anticoagulation was inversely
correlated with recurrence of PVT after discontinuation of treatment
Conflict of interest: none
References:
[1] Ponziani FR, Zocco MA, Garcovich M, D'Aversa
F, Roccarina D, Gasbarrini A. What we should know about portal vein thrombosis in cirrhotic
patients: a changing perspective. World J Gastroenterol. 2012 ;18(36):5014-20.
[2] Zocco MA, Di Stasio E, De Cristofaro R, Novi M, Ainora ME, Ponziani
F and al. Thrombotic risk factors in patients
with liver cirrhosis: correlation with MELD scoring
system and portal vein
thrombosis
development.
J
Hepatol. 2009
;51(4):682-9.
[3] Werner KT, Sando S, Carey EJ, Vargas HE, Byrne TJ, Douglas
DD and al. Portal vein thrombosis in patients
with end stage liver disease
awaiting liver transplantation: outcome of anticoagulation. Dig Dis Sci. 2013 Jun;58(6):1776-80.
[4] Villa E, Camma C, Marietta M, Luongo
M, Critelli R, Colopi S and al. Enoxaparin prevents portal vein thrombosis and liver decompensation in patients
with advanced cirrhosis. Gastroenterology. 2012;143(5):1253-60.
[5] Wanless IR, Liu JJ, Butany J. Role of thrombosis in the pathogenesis of congestive hepatic fibrosis. Hepatology. 1995;21(5):1232-7.
[6] Delgado MG, Seijo S, Yepes I, Achecar
L, Catalina MV, Garcia-Criado A and al. Efficacy
and safety of anticoagulation on patients
with cirrhosis and portal
vein thrombosis. Clin Gastroenterol Hepatol. 2012;10(7):776-83
[7] Amitrano L, Guardascione MA, Menchise
A, Martino R, Scaglione
M, Giovine S andal. Safety and efficacy of anticoagulation therapy with low molecular weight heparin for portal vein thrombosis in patients
with liver cirrhosis. J Clin Gastroenterol. 2010;44(6):448-5
[8] Francoz C, Valla D, Durand F. Portal
vein thrombosis, cirrhosis, and liver transplantation. J Hepatol.
2012 l;57(1):203-12.
[9] Romero-Gomez M, Gutierrez-Tous R, Delgado-Mije D. Anticoagulation therapy for recent portal vein thrombosis in a patient
with liver cirrhosis
suffering from variceal rebleeding. Gastroenterology. 2002;122(7):2095.
[10] Huard G, Bilodeau M. Management of anticoagulation for portal vein thrombosis in individuals with cirrhosis: a systematic review. Int J Hepatol.
2012; 2012:672986.
[11] Valla D. Place des anticoagulants au cours de la cirrhose
[Internet]. 2014. [cited
january21]. Available from: http://www.fmcgastro.org/textes-postus/postu- 2014/place-des-anticoagulants-au-cours-de-la-cirrhose
[12] Senzolo M, T MS, Rossetto
V, Burra P, Cillo U, Boccagni
P andal. Prospective evaluation of anticoagulation and transjugular intrahepatic portosystemic shunt for the management of portal vein thrombosis in cirrhosis. Liver Int. 2012;32(6):919-27
[13] Luca A, Caruso S, Milazzo M, Marrone G, Mamone G, Crino F and al. Natural
course of extrahepatic nonmalignant partial portal vein thrombosis in patients
with cirrhosis. Radiology. 2012;265(1):124-32.
[14] Martinez M, Tandra A, Vuppalanchi R. Treatment
of acute portal vein thrombosis by nontraditional anticoagulation. Hepatology. 2014;60(1):425-6.
[15] Intagliata NM, Northup PG. Anticoagulant Therapy in Patients with Cirrhosis. Semin Thromb Hemost. 2015l;41(5):514-9.
[16] Francoz C, Belghiti
J, Vilgrain V, Sommacale
D, Paradis V, Condat
B, et al. Splanchnic vein thrombosis in candidates for liver transplantation: usefulness of screening
and anticoagulation. Gut. 2005;54(5):691-7.
Citation : Bibani N, Trad D, Bejaoui M, Sabbeh
M, Gargouri D, Elloumi Hela, et al. Non tumoral portal vein thrombosis during
cirrhosis: Should anticoagulation be proposed? Junior Medical Research. 2018;
1(2):4-11. Bibani et al © All rights are reserved. Submit your
manuscript: www.jmedicalresearch.com




